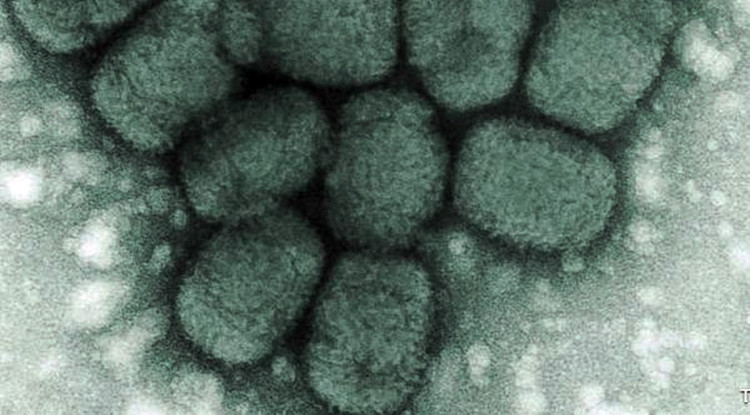
Smallpox was eradicated worldwide in 1980, but the U.S. Food and Drug Administration (FDA) has nevertheless approved the first drug to treat smallpox in the event this deadly virus is used as a biological terror weapon by America's state foes or terrorist groups. This infectious disease killed over 500 million people in the 20th century.
The U.S. Centers for Disease Control and Prevention (CDC) admits there is "credible concern that in the past some countries made the virus into weapons, which may have fallen into the hands of terrorists or other people with criminal intentions."
To counter this future threat, the FDA on July 13 approved the use of "TPOXX (tecovirimat)," a small-molecule antiviral treatment made by SIGA Technologies Inc, after priority review. TPOXX is an orthopoxvirus-specific antiviral indicated for the treatment of human smallpox. It is the first approved treatment specifically indicated for the treatment of this disease.
TPOXX, which was developed by SIGA Technologies of New York, is taken a capsule twice daily for 14 days. SIGA said it's delivered two million treatments that were stockpiled by the federal government.
Tests on monkeys and rabbits infected with a virus similar to smallpox revealed that more than 90 percent survived. The safety of TPOXX was evaluated in 359 healthy human volunteers without a smallpox infection. The most frequently reported side effects were a headache, nausea and abdominal pain, said the FDA.
The approval is the first granted under the Material Threat Countermeasure Priority Review Voucher. The development of TPOXX was partially paid for by the federal government. It falls under the FDA's animal rule, "which allows efficacy findings from adequate and well-controlled animal studies to support an FDA approval when it is not feasible or ethical to conduct efficacy trials in humans."
"Today's approval provides an important milestone in these efforts," said FDA Commissioner Dr. Scott Gottlieb. "This new treatment affords us an additional option should smallpox ever be used as a bioweapon."
The FDA said the approval underscores its "commitment to ensuring that the U.S. is prepared for any public health emergency with timely, safe and effective medical products."