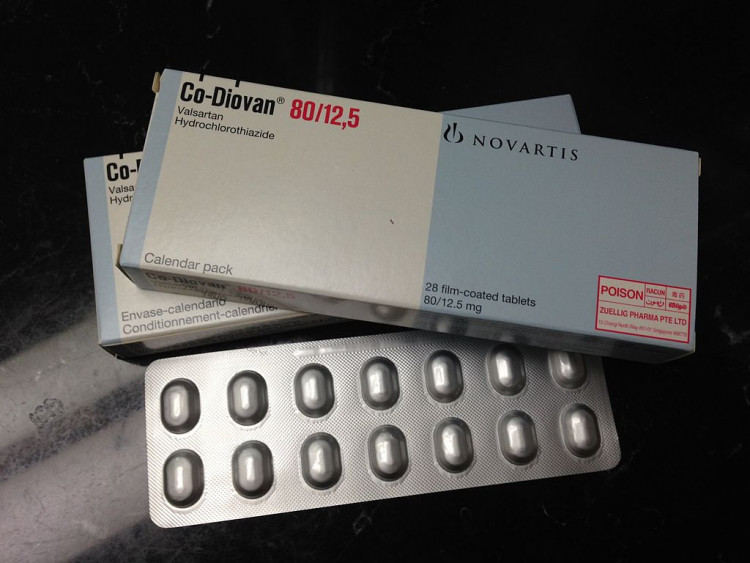
Over 40 different pharmaceutical companies are now facing a wave of new lawsuits centering on the drug valsartan. The drug, widely prescribed as a heart medication, allegedly contains trace amounts of the chemical N-nitrosodimethylamine (NDMA), a known carcinogen. Among the companies that have been targeted by the lawsuits are the drug makers and the distributors of the medication.
According to the US Food and Drug Administration (FDA), which has since issued a recall, millions of Americans take valsartan every day to treat various heart-related ailments, high blood pressure, and other conditions. A slew of class action lawsuits has reportedly now been filed, combining dozens of lawsuits with patients having the same complaints.
A good number of patients have reported come forward, claiming that they had contracted cancer because of taking the now recalled medication. A law firm involved in the filing of the lawsuits revealed that they actually expect at least 2,000 personal-injury cases to be filed in the next two years.
The FDA initially found out about the problem with the particular drug in July of last year. The agency reportedly found out that trace amounts of NDMA were discovered in valsartan that was manufactured by the Chinese company Zhejiang Huahai Pharmaceutical. The drug itself was distributed by the pharmaceutical company to other drug makers, who then used the drug as an ingredient in their other products. Apart from Zhejiang, valsartan produced by other manufacturers have been found to contain NDMA. However, carcinogen levels are reportedly the highest on the drugs produced by the Chinese firm.
Zhejiang Huahai Pharmaceutical was the initial target of the first few lawsuits, but blame has since been distributed to other firms including generic-drugs firm Teva Pharmaceutical Industries. Pharmacies owned by CVS Health have also recently been targeted by litigation. As of the moment, over 40 defendants are facing lawsuits related to the drug.
The FDA revealed that the tainted drug may have been in circulation for as long as four years prior to its NDMA findings. The agency refused to speculate on how many people may have likely developed cancer during that time.
Apart from the victims of the tainted drugs, businesses directly affected by the recall are also reportedly now planning to take legal action. Third-party firms such as health insurance companies are now filing complaints in an attempt to recover economic losses after spending millions of dollars on the tainted products.
Companies such as New York Group Health and SummaCare have already filed suits. Drugs similar to valsartan, such as irbesartan and losartan, have also been recalled by the FDA. However, it is not yet clear if these drugs will be part of the slew of litigations.