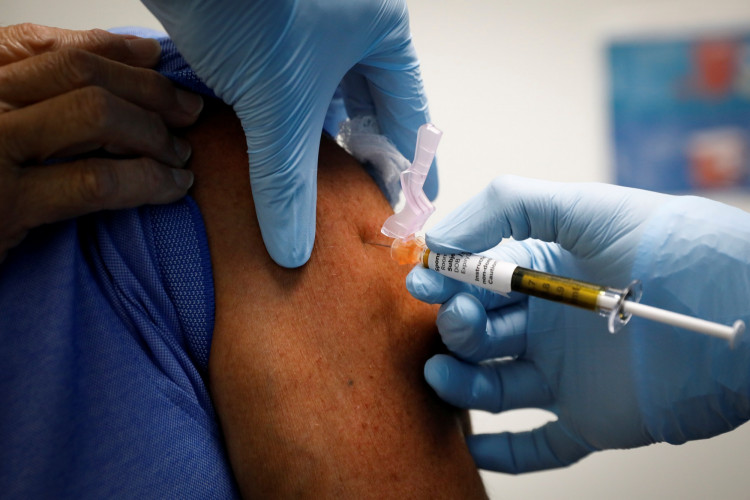
China is rushing to begin vaccinating high-risk groups against COVID-19 as the more dangerous winter months set in and expects to produce more than one billion doses of its three candidate vaccines in 2021.
The start of the country's official vaccination campaign was announced by Zeng Yixin, deputy head of the National Health Commission, on Saturday. Since July, however, China has vaccinated more than a million Chinese unofficially with these three vaccines under emergency use authorization despite their safety and efficacy not being proven by Phase 3 clinical trials.
Zeng said Chinese working in healthcare, customs, wholesale and seafood markets, public transportation, and the cold-chain industry will be among the first to be inoculated. He said that as the temperatures grow much colder in winter and spring, China will have to struggle harder to prevent the spread of COVID-19. Under these conditions, vaccinating key population groups will greatly boost prevention efforts.
Zeng said the upcoming vaccinations are the first step in China's COVID-19 vaccination plan. Vaccination programs for senior citizens, people with underlying diseases, and the general public will be carried out in late 2021 after China's COVID-19 vaccines enter the market and after production ramps-up.
Despite the absence of phase 3 trial data, NHC said the emergency use of the three COVID-19 vaccines showed no severe adverse effects on those inoculated.
In late November, China said it had vaccinated close to a million of its citizens since July with these three vaccine candidates, the largest number anywhere in the world.
The admission was made by Liu Jingzhen, chairman of China National Pharmaceutical Group (Sinopharm), one of whose subsidiaries provided two of the three vaccines used in these inoculations.
Sinopharm's two vaccine candidates were developed by its subsidiary, China National Biotec Group (CNBG). Sinovac Biotech developed the third vaccine.
All three vaccines received emergency use authorization from the NHC. Chinese were inoculated with an inactivated vaccine candidate from CNBG and the CoronaVac vaccine from Sinovac.
Liu claimed there had been no serious adverse reactions to his company's vaccine. He also said Sinopharm had recruited nearly 60,000 people for its Phase 3 clinical trials being undertaken overseas. Blood samples of more than 40,000 participants were taken 14 days after taking the second dose, said Liu.
In September, CNBG said it had inoculated more than 350,000 Chinese outside its clinical trials with the vaccine. The trials have enrolled 40,000 people.
Starting late July, hundreds of thousands of Chinese were inoculated with both vaccines. NHC said these vaccines were administered to medical staff, border inspection officials, and other essential workers.