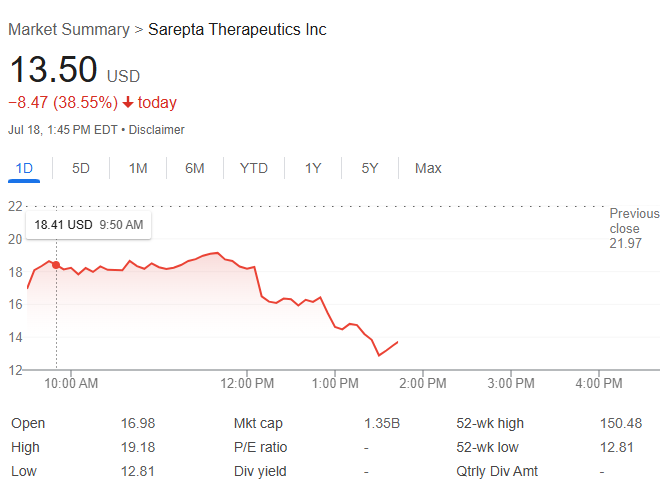
Sarepta Therapeutics saw its shares plunge more than 38% Friday after a third patient death tied to its gene therapy programs deepened regulatory scrutiny and rattled investor confidence. The Food and Drug Administration is reportedly preparing to request a voluntary halt in shipments of Sarepta's approved treatment, Elevidys, according to a person familiar with the matter who spoke to CNBC.
FDA Commissioner Marty Makary confirmed to Bloomberg News that the agency is actively weighing whether Elevidys should remain on the market. The treatment, which was approved to treat Duchenne muscular dystrophy (DMD), has now been linked to two deaths in teenage boys who experienced acute liver failure after receiving the therapy.
Separately, Sarepta confirmed the recent death of a 51-year-old man enrolled in an early-stage trial for SRP-9004, a gene therapy candidate for limb-girdle muscular dystrophy (LGMD). The patient reportedly died from acute liver failure last month. Sarepta stated that liver issues were "not a new safety signal" for the SRP-9004 trial, which has since been halted along with several other LGMD-related programs.
Wall Street analysts warned the mounting safety concerns could undercut physician and patient willingness to use Elevidys, particularly as both therapies rely on the same adeno-associated virus (AAV) vector delivery system. Sarepta disclosed Wednesday that a new label warning would be added to Elevidys regarding acute liver injury risk in ambulatory DMD patients.
The third patient death was first reported by BioCentury Thursday evening, just a day after Sarepta announced it would lay off 500 employees and discontinue multiple gene therapy programs. Sarepta did not mention the death in its Wednesday announcement, a move that William Blair analyst Sami Corwin said could erode investor trust.