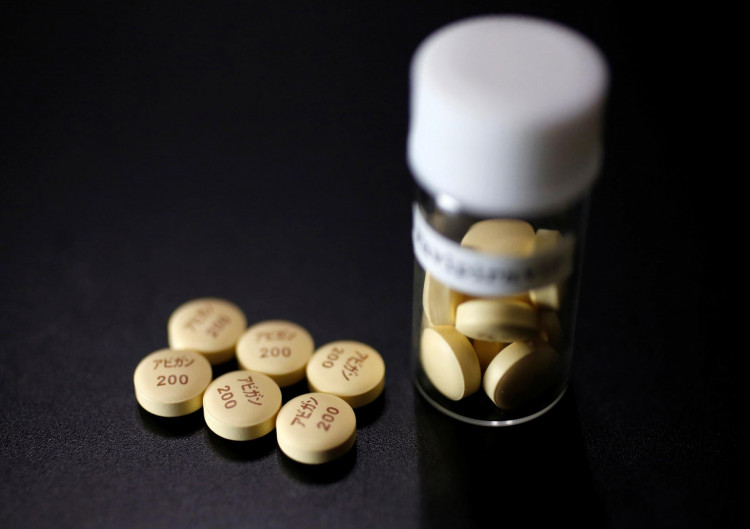
Japan's Fujifilm Holdings Corp, India's Dr Reddy's Laboratories, and Dubai-headquartered Global Response Aid have entered into an agreement to market Fujifilm's COVID-19 anti-influenza vaccine Avigan globally, but with the exception of Japan, Russia and China.
The accord comes as global drug producers race to get manufacturers ready for mass development of possible medication and vaccines for COVID-19, the coronavirus-caused respiratory disease, and move forward with more lab tests.
The Tokyo-based Fujifilm is conducting a clinical test on Avigan to treat COVID-19 patients in the US and Japan and has been collaborating with local and international groups to expand the development of the medication.
Fujifilm will provide Dr Reddy's and GRA a wide inventory of information on Avigan's pre-clinical and clinical research that it has gathered so far while the latter will use it for clinical studies aimed at targeting COVID-19 in areas where coronavirus infections have been rising.
Fujifilm will also grant Dr Reddy's exclusive authorization to use Avigan's formulation patents and production techniques. Dr Reddy's will then create a setup for vaccine production bearing the same quality as Avigan, and use GRA's worldwide sales connection to immediately supply the vaccines in a stable manner.
As part of the deal, Fujifilm would be granted an upfront license fee and royalties based on the sales from Dr Reddy's and GRA. Dr Reddy's had established a partnership with Fujifilm in early 2011 for a generic vaccine business in the Japanese market but both the firms canceled the venture after two years as it did not go as planned.
Russia and India have already given the go signal for the production of Avigan as a COVID-19 treatment, but Japan, whose Prime Minister Shinzo Abe has hailed the vaccine's promise and is optimistic to authorize its use in May, will not come up with a decision until at least this month due to scarcity of patients for clinical tests.
In India, Glenmark Pharmaceuticals Ltd has been given regulatory authorization for favipiravir, the generic form of Avigan, as a therapy for mild to moderate coronavirus infections last June.
Meanwhile, the global magnitude of the coronavirus pandemic is fast rising, the World Health Organization reported, stressing out that last month saw over 50 percent of all cases registered since the beginning of the outbreak.
With more than 511,000 fatalities and more than 10.5 million reported infections globally, the pandemic is not even close to being over, the WHO warned earlier this week.