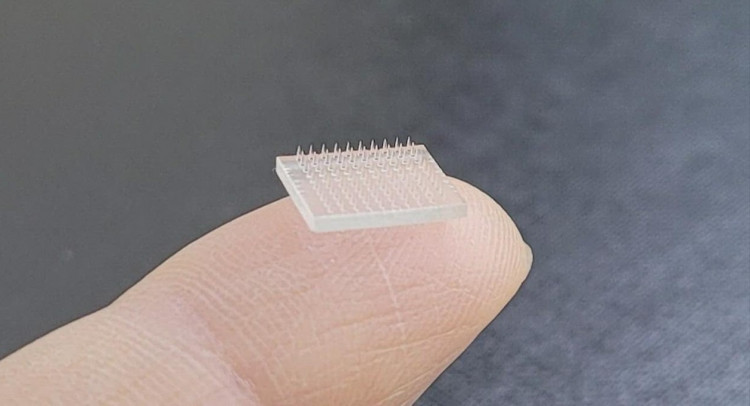
On Wednesday, Swiss medical researchers announced the initiation of an early-stage study to evaluate a next-generation COVID-19 vaccine candidate that would be administered via an arm patch, the latest study to look at alternative methods of administering injections.
Unlike traditional vaccines that boost antibody production, the new PepGNP-Covid19 vaccine candidate concentrates on T-cells, which are responsible for cellular immunity, to kill virus-infected cells and prevent virus replication.
"With this new vaccine that generates this cellular immunity we hope to have a longer period of protection ... we don't know yet, but it could be one year, two years, three years," professor Blaise Genton, head of the study, told Reuters.
The potential vaccine will be injected by micro-needles less than 1mm deep in the patch, which they hope would provide long-term immunity from COVID-19 and eliminate the need for seasonal booster shots.
The potential vaccine was developed by the British company Emergex Vaccines Holding, and the trial, which began on Jan.10, will be administered by the Unisanté medical research center in Lausanne in partnership with the city's CHUV hospital.
To administer the vaccine, the patch will be momentarily pressed against the skin before being removed.
The trial is the first of its kind in the world, and it follows the start of another study in Lausanne last year to test the safety of a new-generation dengue vaccine that uses the same technology.
Vaccines are being developed in new ways by drug companies. A nasal COVID-19 vaccine candidate is being tested by Bharat Biotech, its collaborators Codagenix, and India's Serum Institute.
Last week, the PepGNP-Covid19 researchers began vaccination 26 participants and expect to give them two doses each: a base dose and a slightly higher dose, according to the researchers. The volunteers will be monitored for a period of six months.
Emergex is not the only company working on a COVID-19 vaccine patch; researchers at the University of Texas at Austin have also tested a skin patch with a COVID-19 vaccine candidate dubbed HexaPro.
Despite the fact that it is still in clinical trials, this vaccine has been found to be more heat-resistant than liquid vaccines. When stored at 77F on the patch, it stayed stable for at least one month, and for one week when stored at 104F.
As a result, it is far more suitable for use in locations where many liquid vaccines do not require cold storage. It's also less expensive to make than the vaccines that have already been approved.
If these vaccines are eventually approved, it will be a relief to those who have been unable to get the COVID-19 vaccines due to their fear of needles.