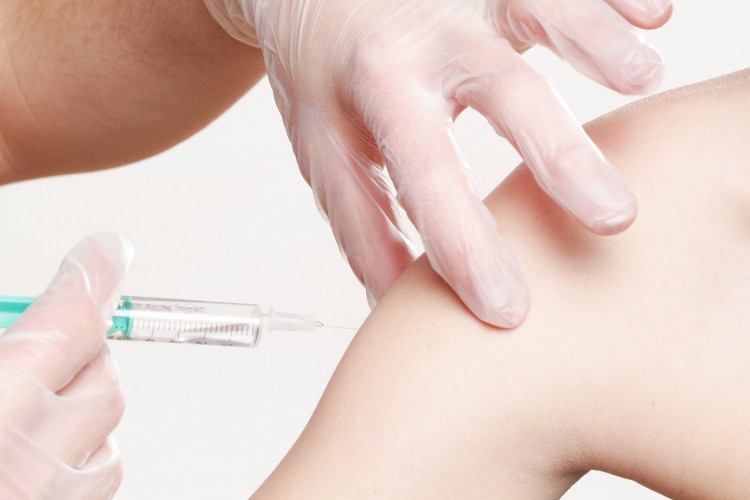
China's State Drug Administration board fined a unit of Changchun ChangSheng Biotechnology $1.3 billion for fabricating its production record of questionable rabies or DPT vaccines that have been administered to hundreds of Chinese babies and children.
The Changchun ChangSheng Life Sciences was first found to have been engaged in malpractice back in July when the government conducted an inspection of its facilities. Specifically, the authorities found that the company fabricated its testing records and production specifications and equipment to hide malicious information regarding the questionable vaccines.
The Associated Press reported that Changchun ChangSheng Life Sciences is now stripped of its license and prohibited from making vaccines and other drugs. China has also banned 14 of its executives from working again in the drugs sector.
Changchun ChangSheng Biotechnology was separately fined $800,000. The company was also banned from participating in the securities market eternally due to its violation of information disclosure regarding the true nature of its vaccines.
In July, CNN reported that the company sold as many as 250,000 faulty vaccines nationwide. At the time, the government estimated that the company still has at least 113,000 doses of the affected rabies vaccines. The majority of these productions was already dispersed in the market and had already been administered to Chinese children under a mandatory national vaccination program.
Some of the unsold vaccines were recalled after the scandal broke out in July. The problem, however, was that there remained to be lack of information regarding the adverse effect of the vaccines that were already injected into children.
Authorities have been concluding that children injected with these faulty vaccines were not actually protected or immune from the disease that the vaccine promised to safeguard them from. Authorities said there is no way to know until they have gathered all those vaccinated for proper monitoring. Any step with regard to the latter would be announced in the future.
Reuters said Changchun ChangSheng Biotechnology has been delaying its announcement of its first-half financial results since July. At the time, the company was saying that it expected its six-month net profit to between $30,000,000 and $45,000,000.
The company's shares have stopped trading since August, a month after the scandal broke out and after ChangSheng Biotechnology failed to release its half-year earning results in due time.
On Oct. 16, the company informed the Shenzhen stock exchange that it is raising separate funds to pay its obligations that resulted from the vaccine scandal. The company also expressed its plans of allotting between $28 million to 90 million to give compensation to the children who have been affected by the faulty vaccines.