The number of reported weekly coronavirus cases globally has declined almost 50% this year, according to the World Health Organization's latest monitoring.
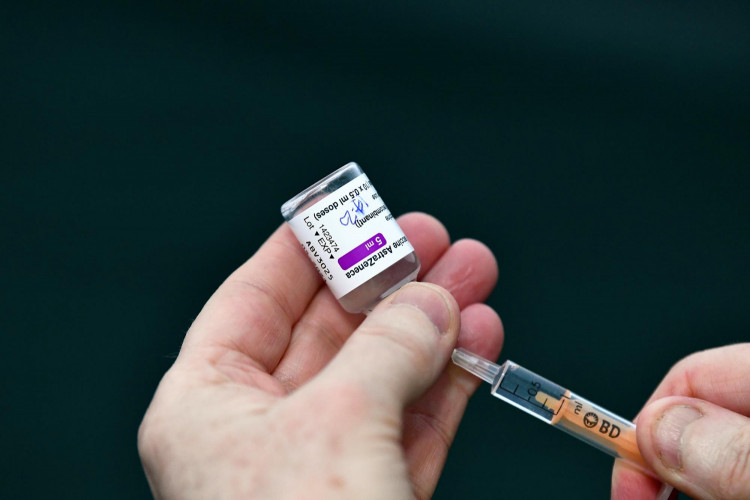
The organization has granted the Oxford/AstraZeneca COVID-19 vaccine an emergency-use listing Monday.
The organization said it "listed two versions of the AstraZeneca-Oxford COVID-19 vaccine for emergency use - giving the green light for these vaccines to be rolled out globally through COVID-19 Vaccines Global Access."
This is the second time the organization has authorized an emergency use listing for a vaccine, the first being for the BioNTech/Pfizer shot Dec. 31.
The COVAX distribution campaign, which is heavily stocked with AstraZeneca vaccines, is scheduled to start gradually dispersing 330 million doses this month.
The two versions that have been granted emergency-use approval are being produced by the Serum Institute of India and AstraZeneca-SKBio of Korea.
The organization emergency-use listing procedure evaluates the safety, quality and effectiveness of COVID-19 vaccines and is a prerequisite for vaccines under the COVAX program.
"Countries with no access to the vaccines to date will finally be able to begin immunizing their health workers and populations at risk," said Dr. Mariangela Simao, organization assistant-director general for access to medicines.
The U.N. health agency has released a vaccine equity declaration that calls for world leaders to increase contributions to the COVAX initiative and to share vaccines with COVAX even as they carry out their own national campaigns.
The organization has also repeatedly urged pharmaceutical companies to submit data in a more timely manner so that it can accelerate listings for a vaccine's emergency use.
Around 145 participating countries are set to be given enough vaccines to inoculate 3.3% of their population by the middle of the year.