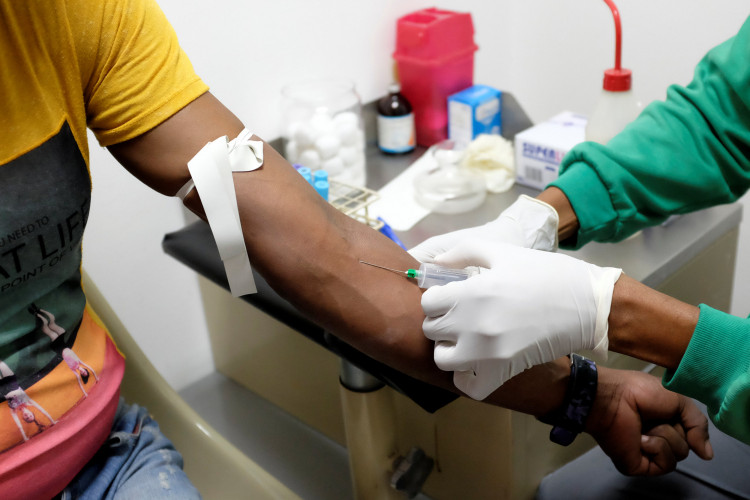
Chinese investigators tested a batch of plasma product, feared to be contaminated with HIV, after the blood plasma scare in China. The result turned negative, and it hoped it would put a stop to another possible medical scandal in the country.
National Medical Products Administration revealed tests for HIV, hepatitis B and C for the batch of 12,000 plasma products made by Shanghai-based China Meheco Xinxing Pharma Co., Ltd. all turned out negative. According to Time, the administration ordered for further examinations and sent its teams after the National Health Commission reported a batch of Meheco-produced plasma products "tested positive for the HIV antibody."
The official Xinhua News Agency also said the products involved are now sealed, and medical institutions all over the country are asked to stop using it as the investigation continues. It is not the first time that China has faced medical scandal. There has been falsifying of production records for rabies, vaccines that resulted in a nationwide crackdown on the industry, and injecting of other faulty vaccines.
This kind of public outrage causes an alarm to the Communist Party's leadership. So, it now responds more "quickly and firmly" compared to the past. It even files criminal charges and billion dollar fines against at fault.
The South China Morning Post added hostile comments about the alleged contaminated blood plasma without knowing the real causes are "unreasonable." The scare would even damage Chinese medical research and the practice's reputation.
China is not the only country that has reported contamination in plasma. Although donated blood undergoes donor selection and screening for HIV-1 and HIV-2, even developed countries have the same problem, according to the medical literature. For an additional measure to reduce the risk of contamination, it is mandatory in Germany to screen "individual donated units and mini-pools" for Hepatitis B RNA.
So the publication advised that before people blame the alleged "profiteers and corrupt practices," the real reason behind the contaminated plasma should be made public. Also, the community has to know any malpractice encountered.
So to assure the public of the plasma products' safety and quality, there should be government reforms on regulations and control. It is also safer to use volunteered blood than paid blood.
A volunteer family donor, on the other hand, may not be as safe as screened blood from the blood bank. "Altruistic" volunteer blood donors are said to be safer than "directed" blood donors like paid donors or the patients' family and friends.