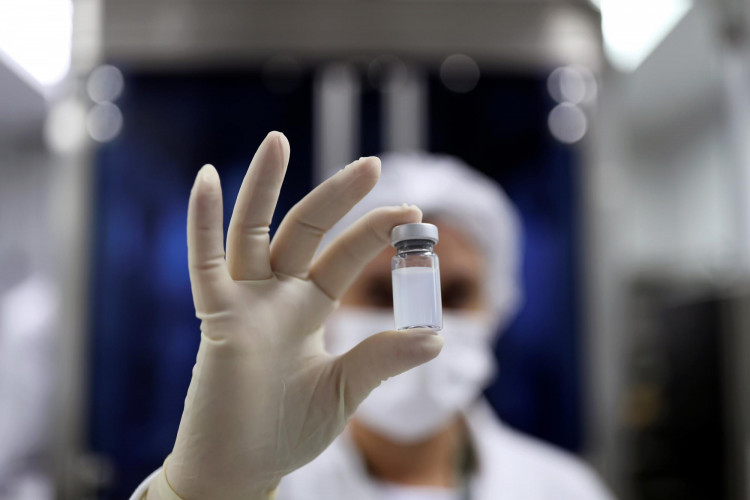
China's drug regulator has given a broader approval for public use to a COVID-19 vaccine developed by the country's Sinovac Biotech, which has faced questions over the effectiveness of its treatment because of lack of transparency on clinical test data.
The approval marks the second vaccine being authorized for general use in the mainland after one produced by a Beijing institute affiliated with government-run China National Pharmaceutical Group (Sinopharm) was given the green light in December.
The vaccine has already been distributed to key groups that are at higher risk of contracting the coronavirus, but Saturday's approval allows for the vaccine's use on a bigger number of people.
The Sinovac vaccine has already been marketed and sold to at least 10 other nations and is being given to patients in at least five other countries. The vaccine was authorized for emergency use last July in China, allowing those in the frontlines to receive it.
From Asia, Africa to Latin America, many developing nations have pinned their hopes on Sinovac's CoronaVac and other Chinese experimental drugs as wealthier countries scramble to secure vaccines produced in the West.
CoronaVac has been found to provide 51% efficacy against COVID-19 during its Brazil clinical test involving 12,397 health personnel older than 18 as of December 16, the company said in a statement.
Sinovac and Sinopharm's vaccines have already been used in China's inoculation campaigns, which have given more than 31 million doses to the public. A fourth potential treatment from CanSino Biologics is being used among the military ranks.
Both vaccines are two-dose inactivated shots that rely on traditional technology, making it easier to transport and store than Pfizer's vaccines, which require super-cold temperatures.
Despite being the first country to begin giving vaccines, China risks being overtaken in the worldwide race to immunize against the disease, a process that could take years in the heavily populated countries of the globe.
Sinopharm will be required to submit further information and reports of any serious side effects after the vaccine is sold on the market.
China has previously disclosed any COVID-19 vaccine will be free for the masses, with the government paying the bill. Sinovac did not immediately give an exact number with regards pricing for each shot.
Meanwhile, Sinovac's Beijing-headquartered arm Sinovac Life Sciences is expected to manufacture more than a billion doses a year in the form of bulk ingredient this month, the company said.